In this informative blog post, we delve into the world of pH control in water. Learn about various substances like calcium carbonate and sodium bicarbonate used to adjust pH levels. Explore equipment such as pH meters and aeration systems for accurate measurement and control. Discover natural materials like limestone and oyster shells for pH modification. Gain insights into biological processes like photosynthesis and denitrification that influence pH. Understand the importance of pH control in industries such as wastewater treatment and food processing. Stay informed about regulations and guidelines, and be aware of health and safety aspects related to pH.
Substances for pH Control: The Magic Ingredients for Chemical Balance
pH, a measure of acidity or alkalinity, plays a crucial role in various natural and industrial processes. Its control is essential to maintain the health of our environment and the safety of our products. Among the tools we have at our disposal are substances that can alter pH, allowing us to fine-tune it to desired levels.
Calcium carbonate, a common substance found in limestone and seashells, is a powerful alkalizing agent. When added to acidic environments, it neutralizes the excess hydrogen ions, raising the pH. This is why calcium carbonate is often used in swimming pools and wastewater treatment plants to prevent corrosion and improve water quality.
Sodium bicarbonate, also known as baking soda, is another versatile pH adjuster. It acts as a weak base, gently raising the pH without drastically shifting the balance. Sodium bicarbonate is commonly found in household products like antacids and baking powder, where it neutralizes acids to alleviate discomfort or create leavening agents.
Other substances, like sodium hydroxide and hydrochloric acid, are stronger alkalis and acids, respectively. These are used in industrial settings to make dramatic pH adjustments. However, due to their corrosive nature, they require careful handling and precise measurements.
Understanding the role of these pH control substances empowers us to manipulate chemical environments to our advantage. By selecting the appropriate substance and adjusting its concentration, we can create optimal conditions for biological processes, prevent equipment damage, and ensure the safety of our products and surroundings.
Equipment for pH Measurement and Control
Maintaining optimal pH levels is crucial in various industries, scientific research, and environmental management. To accurately measure and control pH, specialized equipment plays a vital role.
pH Meters:
pH meters are handheld or benchtop devices that measure the hydrogen ion concentration of a solution. They consist of a glass electrode that generates an electrical potential proportional to the pH. The potential is displayed on a digital or analog scale. pH meters are used in laboratories, water treatment plants, and industrial processes to provide real-time pH readings.
Aeration Systems:
Aeration systems are used to adjust pH by introducing air or oxygen into a solution. Air stones or bubble diffusers are submerged in the solution, releasing countless tiny bubbles. As the bubbles rise, they absorb carbon dioxide from the water, which lowers the carbonic acid concentration and raises the pH. Aeration systems are particularly useful in ponds, aquariums, and wastewater treatment facilities.
Titration Apparatus:
Titration is a technique used to determine the concentration of a solution by adding a known quantity of reagent until a reaction is complete. In pH measurement, titration can be used to determine the acidity or basicity of a solution. Titration apparatus consists of a burette, pipette, and indicator solutions.
pH Controllers:
pH controllers are automated systems that monitor and adjust pH levels automatically. They use sensors to measure the pH and activate pumps or valves to add chemicals or aerate the solution as needed. pH controllers are used in industrial processes, such as chemical manufacturing and wastewater treatment, where precise pH control is essential.
By employing the appropriate equipment for pH measurement and control, industries and researchers can ensure accurate and efficient pH management, leading to optimal performance, environmental protection, and human health.
Natural Materials for pH Adjustment
pH’s Influence on the Environment
pH, measuring acidity or alkalinity, plays a crucial role in various natural processes. The pH of water bodies affects aquatic life, soil fertility, and plant growth. Maintaining optimal pH levels is essential for healthy ecosystems.
Natural pH Modifiers
Nature provides an array of materials that can buffer or adjust pH. Limestone, a sedimentary rock composed primarily of calcium carbonate, is commonly used for this purpose. It raises pH by neutralizing acids and releasing calcium ions into the water column.
Oyster shells offer similar benefits. Rich in calcium carbonate, they slowly dissolve in water, elevating pH over time. Oyster reefs also serve as natural filters, trapping sediment and improving water clarity.
Other naturally occurring pH adjusters include:
- Marl: A soft limestone containing calcium and magnesium carbonates.
- Dolomitic lime: A type of limestone with a higher concentration of magnesium carbonate than calcium carbonate.
- Agricultural lime: A fine powder derived from ground limestone or dolomite, often used for soil pH adjustment.
Benefits of Natural Materials
Using natural materials for pH adjustment has numerous advantages:
- Environmental friendliness: They are naturally occurring and biodegradable, minimizing environmental impact.
- Cost-effectiveness: Natural materials are often readily available and inexpensive compared to chemical additives.
- Long-term effect: They gradually dissolve, providing sustained pH modification over time.
- Multiple benefits: Some materials, like oyster shells, offer additional benefits such as habitat provision and water filtration.
Applications of Natural pH Adjusters
Natural materials find applications in various water management scenarios:
- pH stabilization in ponds and lakes: Buffering pH prevents extreme fluctuations, protecting aquatic organisms and promoting healthy ecosystems.
- Acid neutralization in wastewater: Natural materials neutralize acidic wastewater from industrial or mining operations before releasing it into the environment.
- Soil pH amendment in agriculture: Adjusting soil pH using limestone or dolomitic lime improves nutrient availability, plant growth, and crop yields.
Harnessing the power of natural materials for pH adjustment offers sustainable and cost-effective solutions. Limestone, oyster shells, and other earth’s materials provide versatile means to maintain healthy pH levels in our ecosystems and support the well-being of both flora and fauna. By incorporating these natural wonders into our water management and agricultural practices, we can foster a more balanced and thriving environment.
Biological Processes Influencing pH
In the realm of aquatic environments, where intricate chemical interactions dance, biological processes play a pivotal role in shaping the delicate balance of pH. Among these processes, two stand out: photosynthesis and microbial denitrification. These biological wonders not only sustain life but also leave their mark on the acidity or alkalinity of their surroundings.
Photosynthesis: A Symphony of Alkalinity
Imagine a verdant meadow teeming with lush greenery. As sunlight kisses the leaves, a magical transformation occurs: the miracle of photosynthesis. Through this intricate process, plants and algae harness the sun’s energy to convert carbon dioxide and water into glucose, the building block of life. In a fascinating side effect, photosynthesis releases oxygen into the atmosphere and raises the pH of the surrounding environment. The presence of dissolved carbon dioxide, a weak acid, decreases as it is consumed by plants, resulting in an increase in alkalinity. This pH shift is particularly evident in aquatic ecosystems, where photosynthetic organisms thrive.
Microbial Denitrification: A Balancing Act
In the depths of oxygen-depleted waters, another biological process makes its presence felt: microbial denitrification. This remarkable feat is performed by specialized bacteria that utilize nitrate as an alternative electron acceptor when oxygen is scarce. As they engage in this transformative process, nitrate is converted into nitrogen gas, which escapes into the atmosphere. This biological alchemy has a profound impact on pH: the removal of nitrate ions, which contribute to acidity, leads to an increase in the pH of the environment. Denitrifying bacteria are often found in anaerobic sediments and groundwater, where they play a crucial role in maintaining pH homeostasis.
By understanding the interplay between these biological processes and pH, we gain a deeper appreciation for the intricate dance of life in aquatic ecosystems. From the photosynthetic wonders that paint our planet green to the microbial alchemists that balance the chemical tapestry, these biological marvels shape the very foundation of our watery world.
Applications of pH Control: A Cornerstone in Various Industries
Introduction
pH plays a crucial role in various industries, ensuring optimal processes, product quality, and environmental safety. This blog delves into the significance of pH control, highlighting its applications in wastewater treatment and food processing.
Wastewater Treatment
In wastewater treatment plants, pH control is paramount for maintaining biological balance and facilitating efficient microbial processes. Optimizing pH values allows microorganisms to thrive, effectively removing pollutants and contaminants from wastewater. By controlling pH, plants minimize odor and corrosion while ensuring compliance with environmental regulations.
Food Processing
pH control is a game-changer in food processing, affecting flavor, texture, and safety. In the dairy industry, for instance, pH adjustment ensures the correct texture and flavor of cheese and yogurt while preventing spoilage. In the beverage industry, pH control prevents microbial growth, stabilizes flavor, and ensures compliance with safety standards.
pH control is a fundamental pillar in various industries, driving efficient processes, product quality, and environmental sustainability. By understanding its applications, we can harness its power to optimize performance and safeguard human health and the environment.
Regulations and Guidelines for pH: Ensuring Optimal Levels
Maintaining optimal pH levels is crucial for various industries and applications. To ensure compliance and protect human health, strict regulations and guidelines have been established.
In the drinking water sector, pH plays a vital role in preventing corrosion and microbial contamination. The World Health Organization (WHO) recommends a pH range of 6.5 to 8.5 for drinking water. Exceeding these limits can compromise water quality and pose health risks to consumers.
Industries that handle chemicals are also subject to pH regulations. The United States Environmental Protection Agency (EPA) regulates pH levels in wastewater treatment facilities to ensure efficient removal of pollutants and prevent environmental damage. Specific industries, such as chemical manufacturing, have their own set of pH guidelines tailored to their processes and materials.
pH monitoring is also crucial in food processing. Optimal pH levels are essential for preserving food quality, safety, and shelf life. For instance, the acidity of fruit juices is regulated to prevent spoilage and maintain their nutritional value.
In addition to these specific regulations, general health and safety guidelines apply to handling acidic or alkaline substances. Workers must wear appropriate protective gear, such as goggles and gloves, to prevent exposure to hazardous chemicals. Emergency protocols are also in place to respond to spills or accidents involving pH extremes.
Understanding and complying with pH regulations and guidelines is not only a legal obligation but also a necessary measure to ensure public health, protect the environment, and maintain the integrity of various industries. Adhering to these guidelines ensures the safe and responsible handling of pH-sensitive materials and products.
**Health and Safety Aspects of pH: Ensuring Safe Handling**
pH, a measure of acidity or alkalinity, plays a crucial role in various environments, including our own bodies. While understanding pH is essential, it’s equally important to be aware of the potential hazards associated with acid-base injuries and the precautions necessary for safe handling.
Acid-Base Injuries: Understanding the Dangers
When the skin or body tissues come into contact with strongly acidic or alkaline substances, chemical burns can occur. These injuries can range from mild irritation to severe tissue damage, depending on the concentration and duration of exposure.
- Acid burns: Highly acidic substances, such as battery acid or hydrochloric acid, can cause immediate damage to the skin, resulting in redness, blisters, and deep burns.
- Base burns: Exposure to strong bases, such as lye or sodium hydroxide, can lead to tissue saponification, a process that converts fats into soap and causes deep, penetrating wounds.
Precautions for Safe Handling
To prevent acid-base injuries and ensure safe handling of pH-altering substances, it’s essential to follow these precautions:
- Wear protective gear: Always wear appropriate gloves, eye protection, and clothing when working with acidic or alkaline solutions.
- Use proper ventilation: Ensure adequate ventilation to prevent inhalation of fumes or vapors.
- Dilute solutions: If possible, dilute concentrated substances before handling them.
- Neutralize spills immediately: In case of spills, use an appropriate neutralizing agent, such as baking soda for acids or vinegar for bases, to minimize tissue damage.
- Seek medical attention: If an acid-base injury occurs, seek immediate medical attention, as even mild burns can become severe if not treated promptly.
Understanding Acid-Base Injuries
It’s important to recognize the warning signs of acid-base injuries, including:
- Pain: Intense burning or stinging sensation
- Redness and swelling: Inflammation and discoloration of the skin
- Blisters: Formation of fluid-filled sacs on the skin
- Tissue damage: Deep wounds or ulcers that require specialized treatment
Key Terms and Concepts for pH: Unraveling the Language of Acidity and Alkalinity
Understanding pH is crucial for various scientific disciplines and everyday life, but its technical terms can sometimes be intimidating. To make pH chemistry more accessible, let’s embark on a storytelling journey to define and explain its essential concepts.
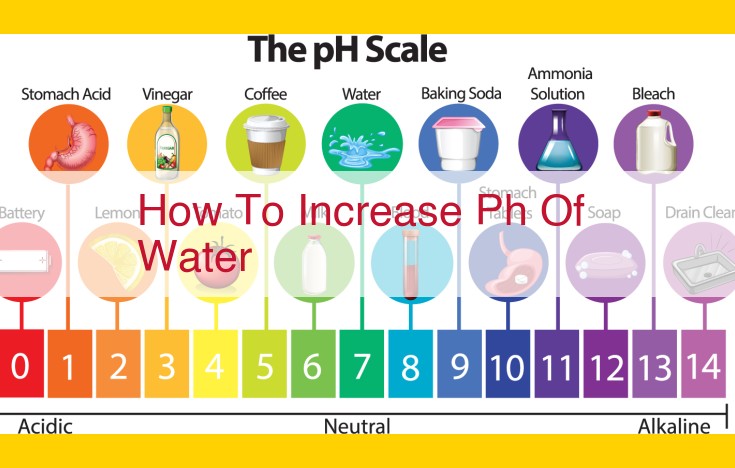
The pH Scale: Measuring Acid-Base Properties
Imagine a mysterious potion with a pH scale, which ranges from 0 to 14. This scale tells us how acidic or alkaline a substance is. Acidic environments have a pH below 7, while alkaline or basic environments have a pH above 7. Neutral substances, like pure water, have a pH of 7.
Alkalinity: The Buffers of Acidity
Meet alkalinity, the unsung hero that helps neutralize acids. It’s like a protective shield, preventing drastic pH changes when acidic substances enter the solution. Substances with high alkalinity, such as bicarbonates and carbonates, are like buffers that soak up excess acidity.
Neutralization: The Balancing Act
Neutralization is a chemical dance where an acid and a base come together to form a salt and water. It’s like a harmonious balance between opposing forces, where the acid’s “sourness” is neutralized by the base’s “bitterness.”
Other Essential Terms
- Acid: A substance that donates hydrogen ions (H+) and lowers pH.
- Base: A substance that accepts hydrogen ions (H+) and raises pH.
- pH Meter: An electronic device that measures the hydrogen ion concentration[H+] of a solution, indicating its pH.
- Titration: A technique used to determine the concentration of an acid or base by adding a known amount of the other.
Understanding these terms will empower you to navigate the world of pH chemistry with confidence. Remember, pH is a fundamental aspect of our environment, influencing everything from the health of our oceans to the efficiency of industrial processes. Embrace these concepts and unravel the secrets of acidity and alkalinity!