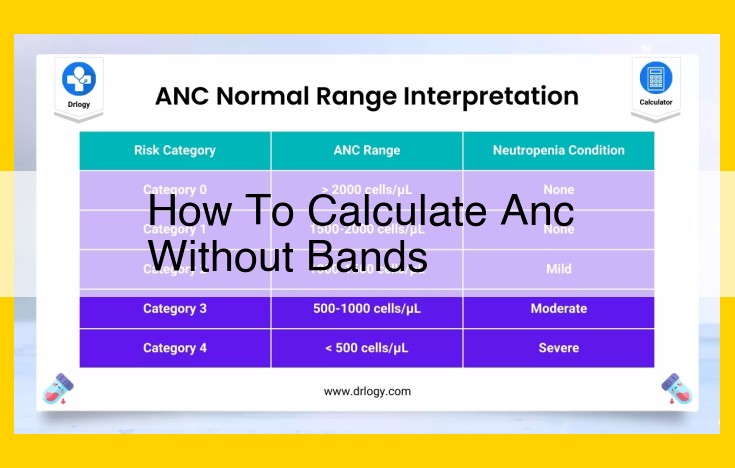
To calculate ANC without bands, gather essential entities like anion capacity (ANC), Gran titration, burette, titrant, and endpoint. Use supporting entities such as phenolphthalein and methyl orange indicators, strong (HCl) and weak (H2SO4) acids, and water quality parameters. Follow steps to perform Gran titration: calibrate burette, prepare sample, and conduct titration. Interpret data using equations and calculate ANC. Consider limitations and error sources.
Calculating ANC Without Bands: A Step-by-Step Guide
Acid Neutralizing Capacity (ANC) is a crucial indicator of water quality. It measures the ability of water to neutralize acids present in the environment. Water with high ANC can effectively buffer against acidic inputs, maintaining a healthy pH level for aquatic life and ecosystems.
Understanding ANC is essential for various applications, including water quality monitoring, wastewater treatment plant operations, and predicting the potential impacts of acid rain or pollution events. This blog post will provide a comprehensive guide to calculating ANC without using titration bands, making it accessible to a wider range of users.
Calculating ANC Without Bands: A Step-by-Step Guide to Water Acidity Assessment
Imagine yourself as a water quality guardian, entrusted with ensuring the purity and health of our precious waters. Among the critical parameters you need to monitor is ANC (Acid Neutralizing Capacity), a measure of water’s ability to resist acidification. Acidic waters can corrode infrastructure, harm aquatic ecosystems, and even pose health risks.
ANC is the buffering capacity of water, its ability to neutralize acids without becoming too acidic. A balance is crucial: high ANC waters are less susceptible to acidification, while low ANC waters are more vulnerable.
Understanding ANC is essential for effective water management. In this blog post, we’ll delve into a simple yet accurate method for calculating ANC without relying on titration bands. Let’s dive right in!
Calculating Acid Neutralizing Capacity (ANC) Without Bands: A Comprehensive Guide
In the realm of water quality assessment, Acid Neutralizing Capacity (ANC) reigns supreme as a crucial parameter. It reflects the water’s ability to resist acidification, maintaining a delicate balance that sustains aquatic life and ecosystem health.
Understanding ANC and Its Significance
ANC is the capacity of water to neutralize acids. It’s a measure of the water’s buffering capacity, its ability to prevent sharp pH swings. A high ANC indicates a water body’s resilience to acidification, while a low ANC suggests vulnerability.
Essential Tools for ANC Calculation
Anion Capacity (ANC): The measure of water’s ability to neutralize acids, expressed in units of milliequivalents per liter (meq/L).
Gran Titration: A precise technique for determining ANC by gradually adding a titrant (acid or base) and measuring the change in pH.
Burette: A graduated glass cylinder with a precision stopcock, used to accurately dispense the titrant.
Titrant: A chemical solution of known concentration used in titration. Commonly, sodium hydroxide (NaOH) is used.
Endpoint: The titration point at which the solution’s pH reaches a specific value.
Supporting Entities for Accurate ANC Calculation
Phenolphthalein Indicator: A color-changing indicator that turns pink at a pH around 8.3, marking a significant transition in ANC calculation.
Methyl Orange Indicator: Another color-changing indicator that turns yellow at a pH around 3.1, signifying a different transition point.
Strong Acid (HCl): A highly concentrated acid used in titration to consume the water’s alkaline components.
Weak Acid (H2SO4): Another acid used to lower the pH of the sample, allowing for a more precise determination of ANC.
Water Quality Parameters: Other parameters such as pH and alkalinity influence ANC calculation and should be considered.
Calculating ANC Without Bands: A Detailed Guide
In the realm of environmental assessment, ANC (Anion Capacity) plays a pivotal role in comprehending water quality. ANC represents the water’s inherent ability to neutralize acids, offering insights into its acidity profile. Comprehending ANC unveils the health of our aquatic ecosystems, and this blog post will guide you through a comprehensive method to calculate ANC without relying on titration bands.
Anion Capacity: The Cornerstone of Calculation
Anion Capacity is the water’s reserve of alkalizing substances, such as carbonates and bicarbonates, which can neutralize acids. The higher the ANC, the more acidic inputs the water can withstand, ensuring the stability of aquatic life.
Gran Titration: A Precise Measurement Technique
Gran titration, a meticulous experimental approach, lies at the heart of ANC calculation. It involves gradually adding a standardized acid to a water sample while monitoring the pH changes. The equivalence point, where the water’s neutralizing capacity is exhausted, provides crucial information for ANC determination.
Essential Tools for Accurate ANC Measurement
To embark on the ANC calculation journey, you’ll need a burette for precise acid dispensing, a titrant (e.g., sodium hydroxide), indicators (phenolphthalein and methyl orange) to detect pH changes, and a strong acid (e.g., hydrochloric acid) for standardization.
Gran Titration: A Precise Way to Measure ANC
In the realm of water quality assessment, Acid Neutralizing Capacity (ANC) holds immense significance. This blog post aims to guide you through the intricate world of ANC calculation, particularly focusing on the Gran titration method, a precise technique to determine ANC without the need for titration bands.
The Essence of Gran Titration
Gran titration, an ingenious method, plays a crucial role in accurately determining ANC. This technique employs a series of incremental additions of a strong base (such as sodium hydroxide) to a water sample while carefully monitoring pH using a pH electrode.
Each addition of base gradually neutralizes the acids present in the sample, causing a corresponding increase in pH. By carefully plotting the pH against the volume of base added and identifying the inflection point, known as the equivalence point, we can determine the ANC accurately.
Benefits and Advantages
The Gran titration method offers several advantages over traditional methods that rely on titration bands. Unlike titration bands, which can be subjective and lead to imprecise results, Gran titration provides _objective and reproducible measurements. It also eliminates the need for costly and time-consuming band preparation, making the process more efficient and cost-effective._
Unveiling the Gran Titration Process
The Gran titration procedure involves a series of steps, each carefully executed to ensure accurate ANC determination. The burette, a precise measuring device, is first calibrated to ensure accurate titrant delivery. The water sample is then prepared and transferred to a titration vessel.
The titration commences with the gradual addition of titrant to the sample while continuously monitoring pH using a pH electrode. The _pH data is plotted against the volume of titrant added, creating a titration curve. The equivalence point, the point at which the maximum rate of pH change occurs, is identified by determining the second derivative of the titration curve._
Calculating ANC from the Gran Titration Data
The ANC value can be calculated using the volume of titrant added up to the equivalence point and the concentration of the titrant. Equations or formulas specific to the titration method and water sample characteristics are employed for the calculation.
It’s important to note that the Gran titration method, while precise, has its limitations and potential sources of error. These factors, including the accuracy of the pH electrode and the presence of interfering substances, should be carefully considered to ensure optimal results.
The Gran titration method is a powerful tool for accurately _determining ANC without relying on titration bands. Its objectivity, reproducibility, and efficiency make it a valuable technique in the realm of water quality assessment. By understanding the principles and steps involved in Gran titration, you can effectively measure ANC and contribute to the preservation of our precious water resources._
Burette: Explain the function and use of a burette for measuring titrant volume.
The Essential Tool for Measuring Titrant Volume: The Burette
In the world of water quality assessment, ANC (Anion Capacity) plays a crucial role in determining the acidity of water. To accurately calculate ANC, a technique called Gran titration is employed. At the heart of Gran titration lies a fundamental tool: the burette.
Imagine yourself standing in a laboratory, armed with a cylindrical glass tube with a calibrated scale etched along its side. This is your burette. Its purpose is simple yet vital: to precisely measure the volume of a titrant, a solution of known concentration that will react with your water sample.
As you carefully pour the titrant from the burette into your sample, you’re engaged in a delicate dance of chemical reactions. The burette acts as your faithful companion, ensuring that the titrant is delivered drop by drop, allowing the reaction to proceed at a controlled pace.
Each drop dispensed from the burette is a testament to the skill of its maker and the precision of the calibration process. The etched scale serves as a guide, allowing you to read the volume of titrant used with meticulous accuracy. It’s through this meticulous measurement that you’ll unravel the secrets of your water sample, uncovering its ANC and shedding light on its acidity level.
Calculating Acid Neutralizing Capacity (ANC) Without Bands: A Comprehensive Guide
Understanding the Acid Neutralizing Capacity (ANC) is crucial for assessing water quality, as it reveals the water’s resilience to acidification. ANC represents the water’s ability to neutralize acids, indicating its buffering capacity. This blog post will guide you through a step-by-step method to calculate ANC without the use of titration bands.
Essential Entities for ANC Calculation
-
Anion Capacity (ANC): ANC refers to the ability of water to neutralize acids, measured in milliequivalents per liter (meq/L).
-
Gran Titration: This technique accurately determines ANC by measuring the volume of titrant required to reach a specific pH endpoint.
-
Burette: A precision instrument used to measure the precise volume of titrant.
Supporting Entities for ANC Calculation
-
Phenolphthalein Indicator: Changes color at a specific pH, indicating the first equivalence point in the titration.
-
Methyl Orange Indicator: Signals the second equivalence point in the titration.
-
Strong Acid (HCI): A known concentration of hydrochloric acid is added to the water sample to lower its pH.
-
Weak Acid (H2SO4): A known concentration of sulfuric acid is used to consume alkalinity in the water sample.
Titrant
The titrant used in this method is typically sodium hydroxide (NaOH), a strong base. The concentration of the titrant is crucial and should be standardized before use.
Steps for Calculating ANC Without Bands
- Calibrate the Burette: Ensure accuracy by calibrating the burette with deionized water.
- Prepare the Sample: Collect a water sample and measure its pH and alkalinity.
- Conduct the Gran Titration: Add the strong acid to the water sample and titrate with NaOH (titrant) using a burette. Record the volume of titrant added at each pH increment.
- Plot the Gran Function: Create a graph of titrant volume (mL) against pH. The Gran function helps identify the equivalence points where ANC is calculated.
Interpretation of Results
- Calculate ANC using the Gran Titration curve and relevant equations.
- Consider the water quality parameters such as pH and alkalinity to assess ANC’s significance.
- Understand the limitations of the method and potential sources of error.
Calculating ANC without bands allows accurate determination of water quality. This method is essential for assessing the buffering capacity of water bodies and understanding their response to acidic inputs. Further research in this area can enhance water quality management practices.
The Elusiveness of the Endpoint: Unveiling the Significance in Titration
In the realm of water quality assessment, titration plays a pivotal role in deciphering the elusive concept of Acid Neutralizing Capacity (ANC). ANC serves as a crucial indicator of a water body’s ability to neutralize acids, thereby safeguarding aquatic life and maintaining ecological balance.
The journey to determine ANC begins with a technique known as Gran titration. This intricate process involves the gradual addition of a precisely measured titrant to a meticulously prepared water sample. As the titrant gently flows into the sample, a subtle chemical dance unfolds. Ions, like invisible protagonists, engage in a series of reactions, their interactions governed by the laws of chemistry.
The endpoint emerges as the defining moment in this titration. It marks the completion of the reaction, a delicate balance where the titrant has neutralized all the acidity within the water sample. Detecting this enigmatic endpoint requires a keen eye and a discerning mind.
Imagine a clear glass beaker containing our precious water sample. A few drops of phenolphthalein indicator, a chemical chameleon, are cautiously introduced. As the titrant continues its steady infusion, the indicator’s hue undergoes a remarkable transformation. It mimics a timid dancer, initially colorless, then blushing a faint pink as the endpoint approaches.
This subtle color shift signals the neutralization of the strong acid component within the sample. The methyl orange indicator, another chemical sentinel, stands ready to reveal the next chapter of this titration tale. It waits patiently for the moment when all the weak acids have succumbed to the titrant’s neutralizing embrace. Its response is equally dramatic, changing from a vibrant orange to a subdued yellow. This second color change marks the true endpoint of the titration, the point where ANC is unveiled.
Grasping the concept of an endpoint is paramount in mastering the art of ANC calculation. It requires precise observation, an understanding of chemical reactions, and a touch of intuitive judgment. With these tools at your disposal, you can unravel the secrets of water quality, safeguarding our precious aquatic ecosystems for generations to come.
The Phenolphthalein Indicator: Unveiling Its Significance in ANC Calculation
The phenolphthalein indicator plays a crucial role in ANC (Acid Neutralizing Capacity) calculation. This indicator changes color at a specific pH level, providing a visual signal that aids in determining the completion of the Gran titration.
Imagine yourself as a chemist performing the titration. You add sodium hydroxide (NaOH) to a water sample, gradually increasing its pH. As the pH rises, various reactions occur, including the release of hydrogen ions (H+). Imagine phenolphthalein as a tiny sentinel, waiting patiently for its moment to shine.
When the pH reaches approximately 8.3, the phenolphthalein molecule undergoes a dramatic transformation. Its colorless form bursts into a vibrant pink hue, indicating that the equivalence point – the point at which the acid has been neutralized – has been reached. This vibrant color change signals that the titration is complete, allowing you to accurately determine the ANC of the water sample.
The phenolphthalein indicator is a crucial tool in ANC calculation because it provides a clear and reliable visual cue. Without it, determining the equivalence point would be much more challenging, leading to less accurate ANC measurements. Hence, the phenolphthalein indicator is an indispensable part of the ANC calculation arsenal, helping ensure precise and reliable water quality assessments.
**Calculating ANC Without Bands: A Simple Guide**
Methyl Orange Indicator: The Key to Accurate ANC Determination
In the realm of water quality assessment, calculating ANC (Anion Capacity) is a crucial measure of acidity and the ability of water to neutralize acidic compounds. To determine ANC accurately, Gran titration is employed, and methyl orange plays a vital role as an indicator.
Methyl orange is a pH-sensitive indicator that undergoes a distinct color change from red to yellow at a specific pH value known as the endpoint. This endpoint corresponds to the neutralization point of the water sample, where the acid and base have reacted completely.
During the titration, methyl orange is added to the water sample. As the base is added, the pH of the solution gradually increases. When the endpoint is reached, the yellow color of the methyl orange indicates that the water has neutralized the added acid and the solution is at a pH of approximately 4.5.
Understanding the role of methyl orange is essential for accurate ANC calculation. By carefully observing the color change at the endpoint, you can precisely determine the amount of acid required to neutralize the water sample. This information, combined with other parameters like anion capacity, Gran titration, and water quality parameters, provides a comprehensive assessment of water quality and its ability to buffer against acidity.
Strong Acid (HCI): Explain the need for a strong acid (e.g., hydrochloric acid) and its concentration in the calculation.
Strong Acid (HCl): The Essential Catalyst for Precise ANC Determination
In the intricate dance of ANC calculation, a strong acid plays the pivotal role of a catalyst, orchestrating the chemical reactions that unveil the elusive acidity of water. Hydrochloric acid (HCl) stands out as the preferred choice, its robust acidity providing the necessary oomph to liberate hidden anions, revealing the true extent of water’s ability to neutralize acids.
The concentration of HCl employed in this delicate procedure is meticulously calibrated to ensure accuracy and precision. A higher concentration enhances the acid’s effectiveness, driving the reaction swiftly towards its endpoint. This surge of acidity ensures that all available anions are swiftly captured, leaving no stone unturned in the quest for ANC determination.
However, the potency of HCl must be carefully balanced to avoid overstepping the boundaries of accuracy. An excessive concentration can lead to false readings, obscuring the true ANC value in a veil of chemical excess. Hence, the choice of HCl concentration is a delicate balancing act, requiring the steady hand of an experienced chemist.
Gran Titration: Understanding the Significance of Weak Acids in ANC Calculation
When it comes to assessing water quality, accurately determining Anion Capacity (ANC) is crucial. ANC measures the ability of water to neutralize acids and is inversely related to water acidity. To calculate ANC without titration bands, the process known as Gran Titration is employed.
In Gran Titration, a strong acid (usually HCI) is added to a water sample. As the acid reacts with carbonate and bicarbonate ions present in the water, Carbonic Acid (H2CO3) is formed. This weak acid then reacts with a weak base (usually H2SO4) to form Na2SO4 and H2O.
The equivalence point in Gran Titration is when H2CO3 is completely neutralized by Na2SO4. At this point, the pH of the solution will be 8.3. This endpoint can be visually detected using Phenolphthalein Indicator, which turns colorless at this pH.
By accurately measuring the volume of strong acid added to reach the equivalence point, the ANC of the water sample can be calculated. The concentration of the weak acid (H2SO4) plays a critical role in this calculation. It must be carefully chosen to ensure that the equivalence point is reached within a reasonable volume of strong acid addition.
Understanding the role of weak acids in Gran Titration is essential for precise ANC determination. This titration technique allows us to assess water quality accurately without relying on titration bands, providing valuable insights for managing water resources and preserving aquatic ecosystems.
Water Quality Parameters: The Pillars of ANC Calculation
When it comes to assessing water quality, Acid Neutralizing Capacity (ANC) plays a pivotal role in understanding its acidity and alkalinity balance. As the name suggests, ANC measures a water sample’s ability to neutralize acids, thus providing valuable information about its environmental health.
Calculating ANC involves a meticulous process that considers various water quality parameters. Among these, pH and alkalinity stand out as essential elements that directly influence the accuracy of ANC measurements.
pH: pH measures the acidity or alkalinity of water on a scale of 0 to 14, where 7 represents neutral. Acidic waters have a pH below 7, while alkaline waters have a pH above 7. ANC is strongly affected by pH, as it influences the availability of ions that contribute to the water’s acid-neutralizing capacity.
Alkalinity: Alkalinity refers to the ability of water to resist pH changes when acids are added. It is primarily determined by the presence of bicarbonate, carbonate, and hydroxide ions. High alkalinity levels indicate a greater capacity to neutralize acids, resulting in higher ANC values.
Therefore, understanding pH and alkalinity is crucial for precise ANC calculation. Accurate measurements of these parameters allow scientists and environmentalists to assess water quality accurately and implement appropriate management strategies to protect our precious water resources.
Calculating Acid Neutralizing Capacity (ANC) Without Bands: A Step-by-Step Guide
ANC, or acid neutralizing capacity, is a measure of the water’s ability to neutralize acids. It is an essential water quality parameter that indicates the water’s buffering capacity against acidification.
Step-by-Step Gran Titration Procedure
Calibrating the Burette:
Start by calibrating the burette with distilled water to ensure accurate volume measurements. Record the initial and final readings of the burette.
Preparing the Sample:
Collect a water sample and measure its volume into a flask. Adjust the pH of the sample below 4.5 using a strong acid like hydrochloric acid.
Conducting the Titration:
Add a weak acid like sulfuric acid to the sample and stir constantly. Use a burette to slowly add a standardized solution of sodium hydroxide to the sample while continuing to stir.
Monitoring the pH:
Use a pH meter to monitor the pH of the sample. Titrate until the pH reaches 8.3. Record the volume of sodium hydroxide added.
Second Titration Point:
Continue titrating until the pH rises to 4.5. Record the total volume of sodium hydroxide added. This volume represents the alkalinity of the sample.
Interpreting the Results
Calculating ANC:
Subtract the alkalinity volume from the total titration volume. The result is the ANC of the water sample. The units of ANC are typically expressed in milligrams of calcium carbonate per liter (mg/L CaCO3).
Limitations and Sources of Error
This method assumes that the water sample contains only carbonate and bicarbonate alkalinity. Other substances, such as organic acids, can interfere with the results.
Potential Errors:
- Incorrect calibration of the burette
- Incomplete mixing of the sample during titration
- Temperature fluctuations can affect the titration results
- Human error in reading and recording data
Despite these limitations, the Gran titration method without bands provides a relatively simple and accurate way to calculate ANC in water samples. Accurate ANC data is critical for assessing water quality, managing acid deposition, and preserving aquatic ecosystems.
Calculating ANC Without Bands: A Step-by-Step Guide
ANC, or Acid Neutralizing Capacity, is a crucial parameter in assessing water quality. It reflects the water’s ability to neutralize acids and maintain a stable pH. Accurate ANC determination is essential for understanding the health of aquatic ecosystems and preventing acidification. This blog post will guide you through a simple method to calculate ANC without using titration bands, making it accessible to a wider audience.
Essential Entities for ANC Calculation
- Anion Capacity (ANC): ANC measures the concentration of anions (negatively charged ions) that can neutralize acids in water.
- Gran Titration: A technique used to accurately determine the ANC by measuring the amount of base required to neutralize sample acidity.
- Burette: A graduated glass tube used to accurately measure the volume of titrant added.
- Titrant: A known concentration of sodium hydroxide solution used to neutralize the sample acidity.
- Endpoint: The point at which the titration is complete, indicated by a color change of the indicator.
Supporting Entities for ANC Calculation
- Phenolphthalein Indicator: An indicator that changes color from colorless to pink at pH 8.3, indicating the presence of weak acids.
- Methyl Orange Indicator: An indicator that changes color from red to yellow at pH 4.5, indicating the presence of strong acids.
- Strong Acid (HCl): A known concentration of hydrochloric acid used to ensure the titration endpoint is reached.
- Weak Acid (H2SO4): A known concentration of sulfuric acid used to calibrate the burette.
- Water Quality Parameters: pH and alkalinity influence ANC calculation and need to be considered.
Steps for Calculating ANC Without Bands
Calibrating the Burette
- Rinse the burette with the titrant.
- Fill the burette with titrant and record the initial reading.
- Dispense 25 mL of the weak acid into a conical flask.
- Add 2-3 drops of methyl orange indicator.
- Slowly add the titrant until the solution turns yellow, indicating the endpoint.
- Repeat the titration with 25 mL of deionized water to account for the acidity of the water used.
- Calculate the burette correction factor using the following formula:
Burette Correction Factor = (mL Weak Acid - mL Water) / mL Titrant
Preparing the Sample
- Collect the water sample in a clean container.
- Determine the pH and alkalinity of the sample.
Conducting the Titration
- Measure 100 mL of the sample into a conical flask.
- Add 2-3 drops of phenolphthalein indicator.
- Slowly add the titrant until the solution turns pink, indicating a pH of 8.3.
- Record the volume of titrant used. This is known as the phenolphthalein endpoint.
- Add 2-3 drops of methyl orange indicator.
- Continue adding the titrant until the solution turns yellow, indicating a pH of 4.5.
- Record the total volume of titrant used, which is known as the total endpoint.
Interpretation of Results
- Calculate the ANC using the following formula:
ANC = (V1 - V2) * C * (1 + F) * 500 / m
- Where:
- V1 = Total volume of titrant used
- V2 = Volume of titrant used to reach the phenolphthalein endpoint
- C = Concentration of titrant (mol/L)
- F = Burette correction factor
- m = Volume of sample used (mL)
Calculating ANC without bands provides an accurate method for assessing water quality. By following the steps outlined in this guide, you can determine ANC and ensure the health of aquatic ecosystems. This knowledge empowers us to make informed decisions and take proactive measures to protect our water resources.
Calculating ANC Without Bands: A Step-by-Step Guide
Understanding how acid neutralizing capacity (ANC) influences water quality is crucial. ANC measures a water body’s ability to neutralize acids and maintain a stable pH level.
To calculate ANC accurately, we’ll embark on a step-by-step journey without relying on titration bands. Let’s dive into the essential entities and supporting entities involved in this process.
Essential Entities
- Anion Capacity (ANC): ANC measures the water’s capacity to neutralize acids. It’s expressed in milligrams per liter (mg/L) of calcium carbonate (CaCO3).
- Gran Titration: A technique that provides accurate and reliable ANC determination. It involves titrating a water sample with a strong base to determine the equivalence point.
- Burette: A graduated glass tube used to measure the precise volume of titrant added.
- Titrant: A standardized solution of known concentration, such as sodium hydroxide (NaOH), used for titration.
- Endpoint: The point at which the reaction between the titrant and the sample is complete. It’s indicated by a change in color of the indicator.
Supporting Entities
- Phenolphthalein Indicator: A colorless compound that turns pink at the equivalence point, indicating the neutralization of weak acids.
- Methyl Orange Indicator: A reddish-orange compound that turns yellow at the equivalence point, indicating the neutralization of strong acids.
- Strong Acid (HCl): Used to calibrate the burette and adjust the sample’s pH before titration.
- Weak Acid (H2SO4): Used to subtract the alkalinity from the total ANC, which provides a more accurate ANC value.
- Water Quality Parameters: pH and alkalinity are important water quality parameters that influence ANC calculation.
Calculating ANC Without Bands: A Comprehensive Guide
In the realm of water quality assessment, Acid Neutralizing Capacity (ANC) plays a crucial role in evaluating the ability of water to neutralize acidity. ANC is a measure of alkalinity and indicates the buffering capacity of water against acidification. This blog post embarks on a journey to outline a method for calculating ANC without relying on titration bands, making it accessible to a broader audience.
Essential Entities for ANC Calculation
At the heart of ANC calculation lies the concept of Anion Capacity (ANC), which represents the molar concentration of weak acid anions in water. Gran titration, a precise technique, enables us to determine ANC accurately. Armed with a burette, we precisely dispense a titrant, typically sodium hydroxide, of known concentration. The titration endpoint, signaled by a change in color of an indicator like phenolphthalein, marks the completion of the reaction.
Supporting Entities for ANC Calculation
Methyl orange, another indicator, plays a pivotal role in ANC determination. Strong acids like hydrochloric acid (HCl) and weak acids like sulfuric acid (H2SO4) also contribute to the calculation. Understanding water quality parameters such as pH and total alkalinity further enhances the accuracy of ANC estimates.
Steps for Calculating ANC Without Bands
Embarking on the step-by-step procedure, we meticulously calibrate the burette and prepare the water sample. With steady hands, we conduct the Gran titration, monitoring the color change of the indicator. The volume of titrant used, carefully noted, holds the key to unlocking the ANC value.
Interpretation of Results
The titration data is a treasure trove of information. We employ equations and formulas to calculate ANC, revealing the buffering capacity of the water sample. Recognition of limitations and potential sources of error in the method is paramount for reliable results.
This blog post has illuminated the intricacies of calculating ANC without bands. Accurate ANC determination is vital for assessing water quality and safeguarding aquatic ecosystems. The ability to perform this calculation empowers individuals to contribute to environmental stewardship and protect the health of our water resources. Let us embrace this knowledge and continue our quest for clean, healthy water for generations to come.
Calculating ANC Without Bands: Limitations and Potential Errors
Methodological Limitations:
- Sampling error: Collecting a representative water sample is crucial, as variations in ANC can occur within water bodies.
- Equipment accuracy: Calibrating and maintaining the burette, pipettes, and other equipment is essential for precise measurements.
- Indicator sensitivity: The accuracy of the ANC calculation depends on the sensitivity of the indicators used (phenolphthalein and methyl orange).
Potential Sources of Error:
1. Incomplete Acid Neutralization:
- If the titration is stopped before all the acid is neutralized, the ANC value will be overestimated.
- To minimize this error, the titration should continue slightly past the equivalence point (indicated by a distinct color change).
2. Overtitration:
- Adding too much titrant past the equivalence point will result in an underestimation of ANC.
- Careful monitoring of the color change and the addition of titrant in small increments are crucial to avoid overtitration.
3. Incorrect Indicator Selection:
- Using the wrong indicator or not observing the color change carefully can lead to inaccurate endpoint determination.
- Fenolphthalein does not change color in the presence of weak acids, while methyl orange changes color too early, resulting in potential errors in ANC calculation.
4. Chemical Interferences:
- The presence of other substances in the water sample, such as iron or manganese, can interfere with the titration process.
- Using a buffer or filtering the sample may help mitigate these interferences.
5. Water Quality Fluctuations:
- ANC can vary over time due to changes in water chemistry, such as biological activity or rainfall events.
- Sampling different locations or at different times may yield varying ANC values, indicating the dynamic nature of water quality.
Mitigating Error:
1. Careful Sampling:
- Collect and handle samples according to standardized protocols to minimize contamination or loss of analytes.
2. Equipment Maintenance:
- Regularly calibrate and maintain equipment to ensure accuracy and precision.
3. Indicator Optimization:
- Choose appropriate indicators and observe color changes meticulously to determine the endpoint correctly.
4. Replicates and Controls:
- Perform multiple titrations on the same sample to check consistency and use a control sample (e.g., a known ANC solution) to validate the method.
5. Data Analysis:
- Consult with experts or consult literature to interpret results correctly and account for potential sources of error.
Unlocking Water Quality Insights: A Comprehensive Guide to Calculating ANC Without Bands
In the realm of water quality assessment, anion capacity (ANC) stands as a crucial parameter, reflecting the water’s ability to neutralize acids. This blog post delves into the intricacies of ANC calculation without relying on the conventional method of using titration bands. We will embark on a journey through the essential and supporting entities involved in this process, guiding you step-by-step through the methodology.
Foundation Stones of ANC Calculation
Understanding the following concepts is paramount for a successful ANC calculation:
-
Anion Capacity (ANC): ANC quantifies the concentration of weak acid anions in water, which can buffer against acidification.
-
Gran Titration: Gran titration is a refined titration technique that eliminates the use of indicators and provides highly precise ANC determinations.
-
Titrant and Burette: The titrant, usually sodium hydroxide, is meticulously added using a burette to neutralize the water sample.
Essential Supporting Elements
Gran titration employs indicators and acids to enhance the accuracy of ANC calculation:
-
Phenolphthalein Indicator: Phenolphthalein marks the first equivalence point, indicating the complete neutralization of weak acids.
-
Methyl Orange Indicator: Methyl orange signals the second equivalence point, denoting the neutralization of dissolved inorganic carbon.
-
Strong Acid (HCl): Hydrochloric acid serves as a strong acid for initial titration, while sulfuric acid (H2SO4) functions as a weak acid for further titration.
The Intricate Process of Calculating ANC Without Bands
The step-by-step procedure involves:
-
Calibrating the burette with a standard solution.
-
Preparing the sample by acidifying it to convert bicarbonate to carbonic acid.
-
Conducting the Gran titration while carefully recording the burette readings at specific pH intervals.
Interpreting the Calculated Values
From the titration data, ANC can be calculated using equations. The results reveal the buffering capacity of the water sample. Understanding potential sources of error and limitations ensures reliable interpretations.
Mastering the art of ANC calculation without bands empowers you with a valuable tool for assessing water quality. This technique provides accurate insights into the water’s capacity to resist acidification, enabling informed decisions in water management and environmental conservation.
Reiterate the importance of accurate ANC calculation.
How to Calculate ANC Without Bands: A Guide for Beginners
Understanding the significance of Acid Neutralizing Capacity (ANC) in water quality assessment is crucial. ANC measures the ability of water to neutralize acid inputs and is closely linked to water acidity. This blog post aims to provide a comprehensive outline for calculating ANC without using titration bands.
The Essential Players
- Anion Capacity (ANC): A measure of the reserve alkalinity in water, determined by the concentration of weak acids.
- Gran Titration: A titration technique that accurately determines ANC by neutralizing weak acids with a strong base.
- Burette: A graduated cylinder used to precisely dispense the titrant.
- Titrant: Typically sodium hydroxide (NaOH) with a known concentration.
- Endpoint: The point in titration when the solution changes color, indicating the completion of the neutralization reaction.
Supporting Cast
- Phenolphthalein Indicator: Indicates the endpoint of the first stage of titration, related to carbonic acid (H2CO3) neutralization.
- Methyl Orange Indicator: Used in the second stage of titration, indicating the endpoint for strong acid (HCl) neutralization.
- Strong Acid (HCl): A known concentration of strong acid added to neutralize carbonate and bicarbonate ions.
- Weak Acid (H2SO4): Used to convert bicarbonate ions (HCO3-) to carbonic acid (H2CO3) for accurate ANC determination.
- Water Quality Parameters: pH and alkalinity are essential considerations in ANC calculation.
Steps to Calculate ANC
- Calibrate the Burette: Ensure accurate volume measurements.
- Prepare the Sample: Add indicators and strong acid to the water sample.
- Conduct the Gran Titration: Gradually add NaOH while recording the volume until the endpoint is reached.
Interpreting Results
- Calculate ANC: Use the titration data and equations to determine ANC.
- Limitations: Consider potential errors related to indicator choice and temperature fluctuations.
The Importance of Accurate ANC
Accurate ANC calculation is essential for assessing water quality and evaluating the impact of acidification. It helps identify sensitive ecosystems and informs decisions on pollution control measures.
This blog post outlined a reliable method for calculating ANC without titration bands, providing a valuable tool for water quality professionals. Understanding the importance of ANC and utilizing appropriate techniques contribute to effective water resource management and environmental protection.
Calculating ANC Without Bands: A Comprehensive Guide
The health of our water bodies is paramount, and assessing their ANC (Anion Capacity) is a crucial indicator. ANC reflects the water’s ability to neutralize acids, preventing potentially harmful acidification. This blog post delves into a practical method for calculating ANC without using titration bands.
Essential Entities
- ANC: Measures the ability of water to neutralize acids and serves as a measure of its alkalinity.
- Gran Titration: A technique used to accurately determine ANC by measuring the amount of acid needed to neutralize the water sample.
- Burette: A precision instrument used to measure the volume of titrant added during the titration.
- Titrant: A solution containing a known concentration of a strong base (e.g., sodium hydroxide).
- Endpoint: The point at which the titration is complete, indicated by a change in the indicator color.
Supporting Entities
- Phenolphthalein Indicator: Changes color at a specific pH, indicating the endpoint for the first part of the titration.
- Methyl Orange Indicator: Used to indicate the endpoint for the second part of the titration.
- Strong Acid (HCl): Required to acidify the water sample before titration.
- Weak Acid (H2SO4): An alternative acid that can be used for the titration.
- Water Quality Parameters: pH and alkalinity are important parameters to consider when calculating ANC.
Steps
- Calibrate the burette to ensure accuracy.
- Prepare the water sample by acidifying it with HCl.
- Perform the Gran titration:
- Add titrant until the phenolphthalein indicator changes color.
- Record the titrant volume and the pH.
- Continue adding titrant until the methyl orange indicator changes color.
- Record the second titrant volume and the pH.
- Calculate ANC using the recorded data and the provided equations.
Interpretation
- The ANC is calculated from the titrant volumes and the water quality parameters.
- The accuracy of the calculation depends on the precision of the measurements and the choice of indicators.
Limitations and Errors
- The method assumes that the water contains only carbonate and bicarbonate alkalinity.
- Other factors, such as dissolved organic matter, can interfere with the accuracy of the calculation.
Applications and Further Research
ANC calculation plays a vital role in water quality assessment. It helps determine the buffering capacity of water bodies, assess the impact of pollution, and develop strategies for water treatment.
Further research is needed to refine the method, investigate ANC’s role in various aquatic systems, and explore the influence of emerging contaminants on water alkalinity. Accurate ANC calculation is essential for protecting our valuable water resources.