As electrons move through the electron transfer chain, they release energy used to pump protons across the inner mitochondrial membrane. This proton gradient drives ATP synthesis through oxidative phosphorylation, where ATP synthase harnesses the flow of protons back into the mitochondrial matrix to phosphorylate ADP and create ATP, the primary energy currency of cells.
Electron Transfer Chain: The Energy Powerhouse of Cells
The Electron Transfer Chain: A Dance of Energy
In the heart of our cells lies a microscopic world of tireless workers, performing an intricate dance that fuels our very existence. This dance is the electron transfer chain, a series of protein complexes that work together to generate the energy our bodies need to function.
NADH and FADH2: The Unsung Heroes of Energy Production
The electron transfer chain begins with two essential energy carriers: NADH (nicotinamide adenine dinucleotide) and FADH2 (flavin adenine dinucleotide). These molecules are like tiny batteries, carrying electrons captured during the breakdown of glucose, the food we eat.
Complex I and II: The Gatekeepers of Electron Flow
As the electron dance unfolds, Complex I (NADH-coenzyme Q oxidoreductase) and Complex II (succinate-coenzyme Q oxidoreductase) act as the gatekeepers of electron flow. NADH and FADH2 pass their captured electrons to these complexes, which then pump protons across the mitochondrial membrane, creating a reservoir of energy.
Complex III: The Transporter of Electrons and Protons
Complex III (ubiquinol-cytochrome c oxidoreductase) gracefully takes over the electron dance, facilitating the transfer of electrons to cytochrome c, a small protein that shuttles them through the electron transfer chain. Simultaneously, Complex III transports more protons across the mitochondrial membrane, further fueling the energy reservoir.
Complex IV: The Orchestrator of Respiration
The dance reaches its climax at Complex IV (cytochrome c oxidase), the final powerhouse of the electron transfer chain. Here, electrons are skillfully passed to oxygen, producing water as a byproduct. This electron transfer fuels a proton gradient across the mitochondrial membrane, the culmination of the energy-generating process of cellular respiration.
Mitochondria: The Powerhouse Within
The electron transfer chain resides within the mitochondria, the energy powerhouses of cells. This enigmatic organelle orchestrates the complex symphony of energy production, ensuring that our cells have the fuel they need to perform their vital functions.
The Electron Transfer Chain: Acellular Powerhouse
In the bustling metropolis of our cells, there exists an intricate network of energy-generating machinery known as the electron transfer chain. This intricate system, nestled within the heart of our mitochondria, plays a pivotal role in powering our cellular activities.
NADH and FADH2: The Electron Carriers
Within this cellular powerhouse, two key molecules, NADH and FADH2, emerge as indispensable electron carriers. These molecules function as trusty couriers, transporting electrons through the electron transfer chain, akin to a relay race where the baton of electrons is passed from one carrier to the next.
NADH and FADH2 originate from two vital metabolic pathways: glycolysis and the citric acid cycle. During glycolysis, the breakdown of glucose yields NADH, while the citric acid cycle produces both NADH and FADH2. These electron-laden molecules then embark on their journey through the electron transfer chain, serving as the fuel that drives cellular respiration.
Complex I and II: Electron Entry Points to the Energy Powerhouse
In the intricate symphony of cellular respiration, the electron transfer chain stands as the conductor, orchestrating the flow of energy that powers our cells. At the heart of this chain, two key players emerge: Complex I and Complex II, the electron entry points that set the stage for the energy-generating dance.
Complex I, known as NADH dehydrogenase, is responsible for receiving electrons from NADH, a molecule brimming with energy harvested from the breakdown of glucose and other nutrients. Like a skilled gatekeeper, Complex I allows these electrons to enter the electron transfer chain, marking the beginning of their journey towards the ultimate goal: ATP production.
Alongside Complex I, Complex II, also known as succinate dehydrogenase, plays a crucial role in the electron transfer chain. Unlike Complex I, which accepts electrons from NADH, Complex II specializes in accepting electrons from succinate, a molecule generated during the citric acid cycle, another essential step in cellular respiration.
These two complexes, Complex I and Complex II, serve as the starting points for the electron transfer chain, where the energy stored in NADH and succinate is harnessed for a symphony of cellular reactions that power our bodies. As electrons flow through these complexes, a cascade of energy-generating events unfold, creating the life-sustaining energy currency known as ATP.
Introduce Complex I (NADH dehydrogenase) and Complex II (succinate dehydrogenase) as the entry points for electrons into the electron transfer chain.
Complex I and Complex II: The Electron Transfer Chain’s Gatekeepers
In the bustling metropolis of the mitochondria, where energy is the currency of life, the electron transfer chain plays a pivotal role. It’s like a sophisticated transportation system, transporting electrons like tiny spark plugs to fuel the cell’s energy production.
Complex I: The NADH Welcome Mat
Complex I, also known as NADH dehydrogenase, stands guard at the entry point of the electron transfer chain. Its primary job is to receive the electron-carrying molecule NADH from previous metabolic processes. Think of NADH as the taxi dropping off energetic passengers at the complex’s doorstep.
Complex II: The Succinate Connection
Meanwhile, Complex II, also known as succinate dehydrogenase, welcomes another electron-rich molecule called succinate into the electron transfer chain. These electrons may come from the citric acid cycle, a metabolic pathway that provides fuel for the electron carriers.
A Team Effort: Electron Transfer Begins
Once NADH and succinate have handed over their precious electrons to Complex I and Complex II, the electron transfer chain’s intricate dance begins. These complexes act as stepping stones, transferring electrons from one complex to another, creating a cascade of energy-releasing reactions that ultimately culminate in the production of ATP, the cell’s energy currency.
C. Complex III: Cytochrome c Reductase
- Describe the function of Complex III in transferring electrons and pumping protons across the inner mitochondrial membrane.
Complex III: The Proton-Pumping Electron Transporter
In the bustling metropolis of the electron transfer chain, Complex III, also known as cytochrome c reductase, plays a pivotal role as the central cog. It receives electrons from ubiquinone and ferries them to cytochrome c, setting the stage for the final electron transfer to oxygen. However, Complex III’s superpowers extend beyond electron transportation. It also functions as a prodigious proton pump, actively translocating protons across the inner mitochondrial membrane.
Imagine a bustling subway station where electrons and protons are the commuters. Complex III acts as a conductor, guiding electrons along a specific route while simultaneously pumping protons against their concentration gradient, creating an electrochemical gradient across the membrane. This gradient serves as a crucial energy reservoir, providing the driving force for the synthesis of ATP, the cell’s primary energy currency.
The architecture of Complex III is a marvel of molecular engineering, composed of multiple protein subunits and various prosthetic groups. These prosthetic groups, including cytochromes b, c1, and c, enable the transfer of electrons through redox reactions. As electrons flow through these prosthetic groups, they undergo a series of reductions and oxidations, releasing energy that is harnessed to power the proton-pumping mechanism.
This intricate dance of electron transfer and proton translocation by Complex III is a crucial step in the electron transfer chain, contributing to the efficient production of ATP. This tireless molecular machine serves as a testament to nature’s ingenuity, ensuring that cells have a continuous supply of energy to fuel their vital functions.
Complex III: The Proton-Pumping Electron Transporter
Nestled deep within the mitochondrial membrane’s labyrinthine folds resides Complex III, a pivotal enzyme complex in the electron transfer chain. Its primary mission is to facilitate the seamless transfer of electrons while simultaneously orchestrating an intricate proton dance across the membrane.
Imagine electrons flowing through Complex III like graceful dancers gliding across the stage. As they pass through, they encounter a series of prosthetic groups, including cytochromes and iron-sulfur clusters, which serve as stepping stones, guiding them towards their next destination.
But Complex III has a secret weapon: it doesn’t just transfer electrons; it’s also a master manipulator of protons. As electrons make their journey through the complex, they trigger a cascade of proton-shuttling events. Like a skilled DJ controlling the flow of music, Complex III regulates the passage of protons across the membrane, creating an electrochemical gradient.
This gradient is the fuel that powers the engine of oxidative phosphorylation, the process by which ATP, the cell’s energy currency, is manufactured. As protons surge down their concentration gradient, they drive the rotation of ATP synthase, an enzyme that harnesses the flow of protons to synthesize ATP from ADP (adenosine diphosphate) and inorganic phosphate.
The intricate dance of electrons and protons within Complex III is a testament to the exquisite choreography of cellular energy production. It’s a symphony of biochemical reactions that powers the myriad of life’s processes, from muscle contractions to brainpower.
Complex IV: The Electron Donor and Proton Pump
In the final stage of the electron transfer chain, Complex IV plays a crucial role in transferring electrons to oxygen and establishing a proton gradient across the inner mitochondrial membrane. This gradient is the driving force behind ATP synthesis, the primary energy currency in cells.
Electron Transfer to Oxygen
Complex IV, also known as cytochrome c oxidase, receives electrons from cytochrome c, a small, mobile electron carrier. These electrons are then transferred to oxygen, resulting in the formation of water.
Cytochrome c → Complex IV → Oxygen → H2O
Proton Pumping
Along with transferring electrons, Complex IV also pumps protons from the mitochondrial matrix into the intermembrane space. This proton movement creates an electrochemical gradient, with a high concentration of protons on the outside of the inner mitochondrial membrane and a low concentration on the inside.
This proton gradient generates a potential energy that drives ATP synthesis by another protein complex, ATP synthase. As protons flow back into the matrix through ATP synthase, their energy is harnessed to phosphorylate ADP (adenosine diphosphate) into ATP (adenosine triphosphate):
ADP + Pi + Proton Gradient → ATP
The Powerhouse of the Cell
The electron transfer chain, including Complex IV, is located within the mitochondria, the energy-producing organelles of cells. The ATP generated by this process powers a wide range of cellular activities, making mitochondria essential for life.
Complex IV, as the final electron acceptor in the electron transfer chain, orchestrates both electron transfer to oxygen and proton pumping. These processes establish the proton gradient that fuels ATP synthesis, providing the energy needed for cellular functions. The vital role of Complex IV underscores its importance as a key player in cellular energy production.
Complex IV: The Final Electron Acceptor in the Electron Transfer Chain
In the intricate symphony of cellular respiration, where energy is harnessed to power life’s processes, the electron transfer chain plays a pivotal role. Complex IV, also known as cytochrome c oxidase, stands as the grand finale of this electron-shuttle system, orchestrating the final transfer of electrons to their ultimate destination: oxygen.
This intricate molecular machine, embedded within the inner mitochondrial membrane, is a maestro of charge transfer. It accepts electrons from cytochrome c, a small protein that ferries electrons from previous complexes in the chain. With meticulous precision, Complex IV then donates these electrons to oxygen, completing the electron transfer cascade.
But Complex IV’s role extends beyond electron transfer. As electrons flow through its protein channels, they undergo a remarkable transformation. Their energy, liberated by the downhill electron gradient, is harnessed to pump protons across the inner mitochondrial membrane. This proton-pumping mechanism establishes a critical gradient, a difference in proton concentration that serves as the driving force for ATP synthesis.
With each electron that Complex IV transfers, it contributes to the buildup of this proton gradient. The protons, eager to return to the mitochondrial matrix, flow back through channels in ATP synthase, an enzyme complex that resides adjacent to Complex IV. As protons surge through these channels, they drive the rotation of ATP synthase’s molecular motor, leading to the synthesis of ATP (adenosine triphosphate), the universal energy currency of cells.
Thus, Complex IV serves as the culmination of the electron transfer chain, the crucial link that completes electron transfer and creates the proton gradient essential for ATP production. Without this molecular marvel, the energy that fuels our cells would remain untapped, and life as we know it would cease to exist.
Mitochondria: The Energy Powerhouse
In the realm of cellular life, the electron transfer chain is a crucial symphony of proteins located within the powerhouse of the cell, the mitochondria. Like an orchestra conductor guiding a masterpiece, the electron transfer chain orchestrates the transfer of electrons to produce the cell’s primary energy currency, ATP.
This energy production process is vital for every living cell. It fuels our muscles, powers our brains, and sustains every bodily function. Understanding the role of mitochondria and the electron transfer chain within them is like unlocking the secret to life’s energy source.
The electron transfer chain is a series of four protein complexes embedded in the inner mitochondrial membrane. Each complex acts like a relay runner, passing electrons from one to another. As electrons flow through this chain, they lose energy, which is harnessed to pump protons across the membrane.
This proton gradient is like a battery, creating a store of energy that can be used to drive the synthesis of ATP. ATP, the universal energy currency of cells, provides the fuel for all cellular processes. This energy is used to power muscle contractions, synthesize proteins, and maintain cell homeostasis.
So, the mitochondria is not just a cellular compartment; it’s the energy-producing powerhouse that sustains life. It’s where the electron transfer chain works its magic, transforming energy from electrons into the fuel that powers our cells.
Explain the location of the electron transfer chain within the mitochondria and its importance in energy production.
Electron Transfer Chain: The Powerhouse Within
In the depths of our cells lies a microscopic powerhouse known as the electron transfer chain. This intricate network resides within the mitochondria, the energy generators of our bodies. Like a symphony of tiny musicians, the electron transfer chain plays a vital role in producing the fuel that powers all our cells: ATP.
Imagine the electron transfer chain as a miniature assembly line, where electrons from nutrient molecules are meticulously passed along a series of protein complexes (labeled Complex I to IV). As electrons journey through these complexes, they undergo a series of energy-releasing reactions like dancers performing a carefully choreographed routine.
The final electron acceptor is oxygen, a molecule we breathe in from the air. When electrons reach the final complex (Complex IV), they team up with oxygen to form water, releasing a significant burst of energy. This energy is harnessed to create a proton gradient across the inner mitochondrial membrane – a difference in acidity levels.
The proton gradient is like a dammed-up river, with protons eager to flow back across the membrane. But here’s where the magic happens: a clever molecular machine called ATP synthase harnesses the power of this gradient to generate ATP. ATP, or adenosine triphosphate, is the primary energy currency of our cells, the fuel that powers everything from muscle contractions to brain activity.
So, there you have it! The electron transfer chain within the mitochondria is the heart and soul of our energy production. It’s a remarkable piece of cellular engineering that allows us to harness the power of nutrients to fuel our bodies and minds.
Unlocking Cellular Energy: The Electron Transfer Chain and ATP, the Energy Currency of Life
Within the depths of our cells, there’s a microscopic powerhouse responsible for fueling our every movement and thought: the electron transfer chain. This intricate pathway is where energy is harvested from food, enabling us to live, breathe, and thrive. Join us on a journey of discovery as we delve into the secrets of this remarkable energy-producing machine.
Meet the Electron Transfer Chain
The electron transfer chain is a series of protein complexes embedded within the inner membrane of mitochondria, the organelles known as the “powerhouses of cells.” Its primary role is to extract energy from electrons, passing them along a conveyor belt of complexes, each performing a specific task.
Electrons Get to Work
Electrons, tiny particles carrying negative charges, are the fuel that drives the electron transfer chain. They originate from NADH and FADH2, molecules that act as electron carriers. NADH and FADH2 are produced during cellular respiration, the process of extracting energy from food.
Complex I and II: The Entry Points
The electron transfer chain begins with two complexes, Complex I (NADH dehydrogenase) and Complex II (succinate dehydrogenase). These complexes act as gateways, allowing electrons from NADH and FADH2 to enter the chain.
Complex III: Proton Pumping Powerhouse
Complex III (cytochrome c reductase) is a key player in the chain. It accepts electrons from Complex I and II and uses their energy to pump protons (H+ ions) across the mitochondrial membrane. This creates a proton gradient, a buildup of protons on one side of the membrane.
Complex IV: The Final Electron Destination
Complex IV (cytochrome c oxidase) is the last stop in the electron transfer chain. It transfers electrons to oxygen, the final electron acceptor, creating water (H2O) as a byproduct. This electron transfer also contributes to the proton gradient.
ATP, the Energy Currency
The proton gradient generated by the electron transfer chain is the key to unlocking cellular energy. This gradient drives the synthesis of ATP, the primary energy currency of cells. ATP is a molecule that stores energy in its chemical bonds. When these bonds are broken, energy is released to power cellular processes.
Oxidative Phosphorylation: Converting Proton Gradient to ATP
Oxidative phosphorylation is the process that converts the proton gradient into ATP. As protons flow back across the membrane, they drive the rotation of a molecular machine called ATP synthase. This rotation powers the synthesis of ATP from ADP (adenosine diphosphate) and inorganic phosphate.
Connecting the Pieces: Citric Acid Cycle and Glycolysis
The electron transfer chain is closely linked to two other metabolic pathways: the citric acid cycle (Krebs cycle) and glycolysis. The citric acid cycle provides substrates for NADH and FADH2, while glycolysis generates pyruvate, which is further processed in the citric acid cycle.
The electron transfer chain, together with ATP, is the linchpin of cellular energy production. These intricate pathways ensure that our cells have the fuel they need to perform countless essential functions, from muscle contraction to brain activity. Understanding these processes is not only fascinating but also crucial for our overall health and well-being.
Unveiling the Energy Powerhouse: The Electron Transfer Chain and ATP Production
Every living being, from the tiniest microbe to the mightiest whale, relies on the continuous production of energy to fuel life’s processes. At the heart of this intricate energy-generating machinery lies the electron transfer chain, a complex network of proteins that orchestrate a dance of electrons to produce ATP, the primary energy currency of cells.
The Electron Transfer Chain
The electron transfer chain, nestled within the mitochondria, the powerhouses of cells, is a master of manipulating electrons. Its intricate structure features five protein complexes, each playing a vital role in the electron transfer process.
- NADH and FADH2: The Electron Carriers
NADH and FADH2, two key players, carry electrons that are generated during cellular respiration, the process of breaking down food to produce energy.
- Complex I and II: Electron Entry Points
Complex I and II serve as entry points for electrons into the chain. Complex I accepts electrons from NADH, while Complex II accepts electrons from FADH2.
- Complex III: Cytochrome c Reductase
Complex III, a pivotal component, transfers electrons and pumps protons across the inner mitochondrial membrane. This proton pumping creates a concentration gradient, a driving force for energy production.
- Complex IV: Cytochrome c Oxidase
The final stage, Complex IV, accepts electrons and transfers them to oxygen, creating a proton gradient and water as a byproduct.
Energy Production: Oxidative Phosphorylation
The electron transfer chain serves as the foundation for oxidative phosphorylation, the process by which the proton gradient is harnessed to produce ATP. The ATP synthase enzyme, embedded in the mitochondrial membrane, captures the energy of the proton gradient and converts it into chemical energy stored in ATP.
ATP: The Cellular Energizer
ATP, the adenosine triphosphate, is the universal energy currency of cells. Its unique chemical structure allows it to release energy rapidly and efficiently when it donates its terminal phosphate group. This stored energy powers a vast array of cellular processes, from muscle contraction to nerve transmission.
Related Processes
The electron transfer chain does not operate in isolation. It is intricately connected to other metabolic pathways that provide the substrates it needs to generate electrons.
- Citric Acid Cycle (Krebs Cycle): Electron Carrier Supplier
The citric acid cycle provides the bulk of the electrons for the electron transfer chain. This cycle generates NADH and FADH2, which carry electrons into the chain.
- Glycolysis: The Prelude to Electron Transfer
Glycolysis, the initial step in cellular respiration, produces pyruvate. Pyruvate is then processed in the citric acid cycle to generate the electron carriers NADH and FADH2.
The electron transfer chain and ATP production are essential processes that underpin the very foundation of life. By harnessing the energy released from electrons, cells generate the ATP they need to power the countless processes that keep them alive and thriving. Understanding this intricate machinery provides a glimpse into the remarkable complexity and efficiency of nature’s energy-generating systems.
**Electron Transfer Chain: The Powerhouse of the Cell**
B. Oxidative Phosphorylation: Converting Proton Gradient to ATP
As electrons flow through the electron transfer chain, they create a proton gradient across the inner mitochondrial membrane. This gradient is a source of potential energy, much like a dammed-up river.
Harnessing this energy, a molecular machine called ATP synthase steps into action. It acts like a tiny turbine, allowing protons to flow back through the membrane, driving the rotation of its central shaft.
As the shaft spins, it powers a chemical reaction, converting ADP (adenosine diphosphate) into ATP (adenosine triphosphate). ATP is the cellular energy currency, the fuel that powers all essential cellular processes.
The proton gradient generated by the electron transfer chain is thus essential for ATP synthesis. This is known as oxidative phosphorylation. It’s like a cellular power plant, using the energy of electron transfer to create the currency that drives life.
Additional Points for SEO Optimization:
- Oxidative phosphorylation is a key process in cellular respiration, producing most of the ATP used by cells.
- The electron transfer chain and oxidative phosphorylation are central to mitochondrial function and energy metabolism.
- Dysfunctions in oxidative phosphorylation can lead to various diseases, including mitochondrial disorders.
The Dance of Electrons: Unraveling the Mysteries of Energy Production
In the bustling metropolis of the cell, there’s an intricate dance taking place, a dance that fuels life itself. The electron transfer chain, a series of protein complexes tucked away in the mitochondria, is the maestro of this dance, orchestrating the production of energy that powers our every move.
Join the Electron Symphony
The electron transfer chain, like a finely tuned orchestra, has its own cast of characters:
-
NADH and FADH2: These molecules, the electron carriers, are the spark plugs of the show. They dance with electrons, bringing them to the party.
-
Complex I and II: These complexes act as the gateways, allowing electrons to enter the electron transfer chain.
-
Complex III: This maestro coordinates the transfer of electrons and pumps protons across the inner mitochondrial membrane, like a heartbeat regulating the energy flow.
-
Complex IV: The grand finale! This complex transfers electrons to oxygen, creating a rush of protons that drive energy production.
The Powerhouse in Action
The electron transfer chain doesn’t just shuffle electrons for fun; it’s all about generating energy. This energy comes in the form of ATP, the cell’s universal currency.
As electrons dance through the chain, they create a proton gradient across the inner mitochondrial membrane. This gradient is like a battery, storing energy that can be harnessed to synthesize ATP.
The Magic of Oxidative Phosphorylation
Here’s where the real magic happens: oxidative phosphorylation. This process harnesses the power of the proton gradient to create ATP. It’s like a waterwheel, using the flow of protons to turn ATP-generating machinery.
The Supporting Cast
The electron transfer chain doesn’t work in isolation. It’s part of a larger team:
-
Citric acid cycle (Krebs cycle): This process generates the substrates, NADH and FADH2, that fuel the electron transfer chain.
-
Glycolysis: The prelude to the show, glycolysis prepares the stage by producing pyruvate, which is then processed in the Krebs cycle.
So, as you go about your day, remember the bustling metropolis of your cells, where electrons dance and protons pump, powering the symphony that is life. The electron transfer chain, a marvel of nature, is the conductor of this energy-generating orchestra, ensuring you have the fuel to live, breathe, and thrive.
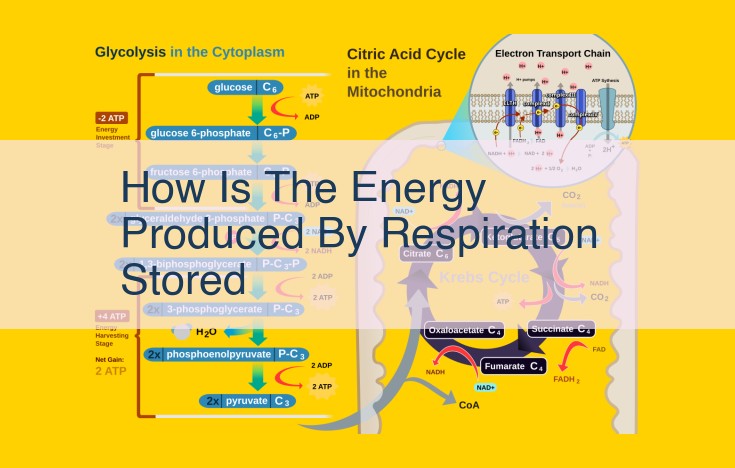
A. Citric Acid Cycle (Krebs Cycle): Substrate for Electron Carriers
- Explain the connection between the citric acid cycle and the electron transfer chain, as the citric acid cycle provides substrates for NADH and FADH2.
The Electron Transfer Chain: Energy Production and the Citric Acid Cycle
The Citric Acid Cycle: Fueling the Electron Transfer Chain
Within our cells, the electron transfer chain plays a crucial role in energy production. This intricate chain is like a symphony of molecules, each with a specific task in generating the ATP—the energy currency of our bodies. A vital partner in this process is the citric acid cycle, also known as the Krebs cycle.
The citric acid cycle is a series of chemical reactions that break down glucose, the sugar we obtain from food. As glucose is processed through the cycle, two important electron carriers, NADH and FADH2, are produced. These molecules are like shuttle buses, carrying electrons to the electron transfer chain.
Electron Transfer Chain: A Power-Generating Symphony
The electron transfer chain resembles a cascade of waterfalls, where each step releases energy. As electrons flow through the chain, they pass through several protein complexes that pump protons across a membrane. This creates a concentration gradient, similar to a battery, with more protons on one side and fewer on the other.
The proton gradient is then used to drive the synthesis of ATP through a process called oxidative phosphorylation. Just as a water turbine harnesses the energy of a waterfall to generate electricity, the proton gradient powers ATP production. This ATP is then used to fuel various cellular processes, providing the energy for our daily activities.
Glycolysis: The Prelude to Energy Production
Before glucose can enter the citric acid cycle, it undergoes a preparatory stage called glycolysis. Glycolysis occurs in the cytoplasm and breaks down glucose into smaller molecules, including pyruvate. Pyruvate then enters the mitochondria, the energy powerhouses of our cells, where it is further processed by the citric acid cycle.
The electron transfer chain and the citric acid cycle work in tandem to produce the energy that powers our bodies. The electron transfer chain is like a conveyor belt, transporting electrons from NADH and FADH2 to create a proton gradient. This gradient is then used to synthesize ATP, which is the energy currency of our cells. The citric acid cycle, in turn, provides the electron carriers with the necessary fuel to keep the electron transfer chain running. Together, these processes orchestrate the continuous generation of energy within our cells.
The Electron Transfer Chain: The Powerhouse of Energy Production
In the heart of our cells, a remarkable molecular machine known as the electron transfer chain orchestrates a symphony of energy conversion. This intricate system plays a crucial role in cellular respiration, the process by which our bodies convert food into usable energy.
The Electron Transfer Chain: Gatekeepers of Cellular Energy
The electron transfer chain is a series of protein complexes embedded in the inner membrane of mitochondria, the powerhouses of our cells. These complexes act as electron carriers, passing electrons along a chain like a relay race. As electrons move through the chain, they lose energy and release protons, creating a concentration gradient across the mitochondrial membrane.
The electron transfer chain consists of four main complexes: Complex I, Complex II, Complex III, and Complex IV. Complex I and Complex II receive electrons from NADH and FADH2, respectively, two molecules generated during cellular respiration. These electrons are then passed along to Complex III and Complex IV, which ultimately transfer them to oxygen, the final electron acceptor.
Energy Production: Harnessing the Proton Gradient
The electron transfer chain sets the stage for oxidative phosphorylation, the process by which the cell generates ATP, the body’s primary energy currency. As electrons move through the chain, they create a proton gradient across the mitochondrial membrane. This gradient is like a tiny battery, storing the energy released by the electron transfer process.
The energy stored in the proton gradient is harnessed by a protein complex called ATP synthase. ATP synthase uses the energy to pump protons back across the mitochondrial membrane, driving the synthesis of ATP from ADP (adenosine diphosphate). This process is essential for powering and maintaining the cell’s vital processes.
Connecting the Dots: The Citric Acid Cycle and Glycolysis
The electron transfer chain is closely linked to the citric acid cycle (Krebs cycle) and glycolysis, two other crucial pathways in cellular respiration. The citric acid cycle provides the substrates, NADH and FADH2, that donate electrons to the electron transfer chain. Glycolysis, which occurs outside the mitochondria, generates pyruvate, which is then processed in the citric acid cycle.
Together, glycolysis, the citric acid cycle, and the electron transfer chain form an integrated system that converts the chemical energy stored in food into usable energy for the cell. This intricate dance of molecular machines fuels the countless biochemical processes that sustain life itself.
Glycolysis: The Prologue to Electron Transfer
In the grand symphony of cellular energy production, the electron transfer chain plays an indispensable role, akin to the conductor orchestrating a harmonious melody. But the electron transfer chain does not operate in isolation. It is intricately entwined with other cellular processes, including the citric acid cycle and glycolysis, which sets the stage for the electron transfer chain’s energetic performance.
Glycolysis, a metabolic dance, takes place in the cytoplasm, the bustling hub of the cell. It is the prelude to electron transfer, a vital process that generates pyruvate, a three-carbon molecule. This pyruvate molecule is not an end in itself but rather an essential substrate for the citric acid cycle, the next act in the energy production play.
As glucose, the body’s primary fuel source, enters the glycolytic pathway, it undergoes a series of enzymatic transformations, each step akin to a graceful dance move. In this metabolic ballet, glucose is broken down into two pyruvate molecules. Not only does glycolysis provide pyruvate for the citric acid cycle, but it also generates crucial electron carriers, NADH and FADH2.
These electron carriers, NADH and FADH2, are the messengers of the electron transfer chain. They carry the high-energy electrons that will power the electron transfer chain’s proton-pumping prowess. Without glycolysis’s diligent generation of NADH and FADH2, the electron transfer chain would be a silent symphony, unable to produce the energy that fuels cellular life.
Thus, glycolysis serves as the prelude to electron transfer, providing the essential substrate and electron carriers that enable the electron transfer chain to perform its energetic opus. In the concert of cellular respiration, glycolysis is the overture, setting the stage for the electron transfer chain’s virtuoso performance.
Electron Transfer Chain: The Powerhouse of Cells
So, What’s the Big Deal?
In the realm of cellular energy production, the electron transfer chain stands as a true powerhouse. This intricate system works wonders to generate ATP, the body’s primary energy currency.
Meet the Key Players
The electron transfer chain, residing within the mitochondria, involves a team of molecular players. NADH and FADH2 take center stage as the electron carriers, shuttling these tiny energy messengers through the chain.
The Electron Highway
The journey begins at Complex I and Complex II, where electrons from NADH and FADH2, respectively, enter the chain. They then make their way through Complex III, which pumps protons across the mitochondrial membrane, creating an energy gradient like a microscopic battery.
The Grand Finale: Complex IV
The grand finale occurs at Complex IV. Here, electrons meet their destiny with oxygen, sparking a chemical reaction that releases energy. This energy drives the creation of a further proton gradient, amplifying the battery’s power.
ATP: The Energy Goldmine
Now, here’s where the magic happens. The proton gradient is like a flowing river, and ATP synthase, a molecular machine, taps into this energy flow to generate ATP. Think of it as a miniature hydroelectric dam, converting the proton gradient’s potential energy into the chemical energy of ATP.
The Connected World of Energy
But the electron transfer chain doesn’t operate in isolation. It’s intricately linked to two other cellular processes:
- Citric Acid Cycle: This cycle provides NADH and FADH2 with the electrons they carry.
- Glycolysis: The starting point of cellular respiration, glycolysis generates pyruvate, which is then processed in the citric acid cycle to produce those crucial electron carriers.
So, there you have it—the electron transfer chain, the beating heart of cellular energy production. It’s a mesmerizing dance of electrons, protons, and molecules, all working in harmony to keep our bodies humming with life.